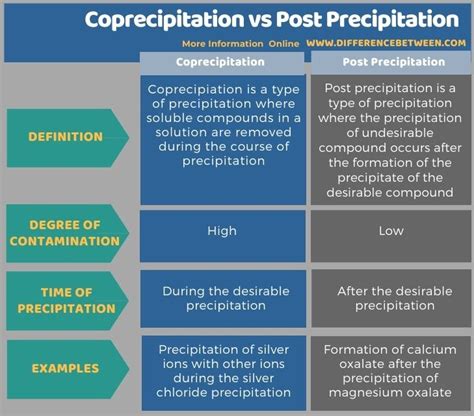
gravimetric analysis precipitation method|types of coprecipitation : dealers All precipitation gravimetric analyses share two important attributes. First, the precipitate must be of low solubility, of high purity, and of known composition if its mass is to reflect accurately the analyte’s mass. . See more Welcome to Casino at bet365. There is a range of games including Blackjack and Roulette. You can register in order to explore the Casino.
{plog:ftitle_list}
web09-Мар-18 08:54 (5 лет 11 месяцев назад, ред. 05-Апр-21 09:14) Rush / Discography. Жанр: Classic Rock, Hard Rock, Progressive Rock, Heavy Prog, Art Rock. Страна: .
In precipitation gravimetry an insoluble compound forms when we add a precipitating reagent, or precipitant, to a solution that contains our analyte. In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate . See more
how to test new hard drive reliability
All precipitation gravimetric analyses share two important attributes. First, the precipitate must be of low solubility, of high purity, and of known composition if its mass is to reflect accurately the analyte’s mass. . See moreAlthough no longer a common analytical technique, precipitation gravimetry still provides a reliable approach for assessing the accuracy of other methods of analysis, or for verifying the composition of standard . See moreAll precipitation gravimetric analysis share two important attributes. First, the precipitate must be of low solubility, of high purity, and of known composition if its mass is to accurately reflect the .
Gravimetric analysis is a quantitative method for accurately determining the amount of a substance by selective precipitation of the substance from an aqueous .Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. Some authors distinguish two sorts of gravimetry by precipitation: direct gravimetry, in which the analyte itself is weighed. For example, when nickel is determined by .
Precipitation gravimetric analysis separates ions from a solution by using the precipitation process (the reaction that creates an insoluble solid product from the reaction of two soluble solid products).
Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate .
Precipitation gravimetry is an analytical technique that uses the formation and mass of a precipitate to determine the mass of an analyte. It is important for the precipitate to be a .
Gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample .The precipitation method is the one used for the determination of the amount of calcium in water. Using this method, an excess of oxalic acid, H 2 C 2 O 4, is added to a measured, . High Sensitivity and accuracy of gravimetric method; Errors in Gravimetric analysis and Precautions. Errors in gravimetric analysis arise due to inaccurate weighing, incomplete and faulty precipitation, incomplete .
Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction. Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . When the signal is the mass of a precipitate, we call the method precipitation gravimetry. . An accurate .
Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed . Gravimetry is a method of quantitative chemical analysis. It qualifies as a macroscopic quantitative method of analysis because it involves relatively important quantities of a substance to be determined compared to more recent methods, such as electrochemical, spectroscopic and chromatographic means.Also common are gravimetric techniques in which the analyte is subjected to a precipitation reaction of the sort described earlier in this chapter. The precipitate is typically isolated from the reaction mixture by filtration, carefully dried, and then weighed (Figure \(\PageIndex{2}\)). . Combustion analysis is a gravimetric method used to . Example 1: Explaining Why Ashless Filter Paper Is Used in Gravimetric Analysis. In the precipitation method, why do we use ashless filter paper in chemical analysis? . As the name suggests, the precipitation method involves reacting an aqueous solution of our analyte with another aqueous solution to produce a precipitate. The precipitate that .
Gravimetric analysis is the process of isolating and measuring the weight of a particular element or compound. The major part of the gravimetric determination of compounds involves the .
Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participate in a chemical reaction. In a direct precipitation gravimetric analysis, for example, we convert a soluble analyte into an insoluble form that precipitates from solution.
types of coprecipitation
Four primary gravimetric analysis methods are used to determine an analyte's mass. Outside of precipitation gravimetry, other common methods include volatilization gravimetry, electrogravimetry .The four main types of this method of analysis are precipitation, volitilization, electro-analytical and miscellaneous physical method. . several organic functional groups or heteroatoms can be determined using gravimetric precipitation methods • Examples are outlined in Table 8.5 Example 1 • An ore containing magnetite, Fe3O4, was .It plays a crucial role in quantitative analysis, where precise measurements are essential for accurate results. By allowing chemists to determine the mass of samples with exceptional accuracy, analytical balances support various methods of gravimetric analysis, including precipitation and volatilization techniques.
In many cases the precipitate includes the analyte; however, an indirect analysis in which the analyte causes the precipitation of another compound also is possible. Precipitation gravimetric procedures must be carefully controlled to produce precipitates that are easy to filter, free from impurities, and of known stoichiometry.Gravimetric analysis is a method in analytical chemistry that uses mass measurement to quantify the quantity of an analyte (the ion being studied). The masses of two substances containing the analyte are compared in gravimetric studies. . Precipitation Method. In Precipitation Gravimetry, a known quantity of the substance that is to be . 4. Types of Gravimetric Analyses: • There are two main types of gravimetric analyses: A) Precipitation – analyte must first be converted to a solid (precipitate) by precipitation with an appropriate reagent. The .Steps of a gravimetric analysis: precipitation, digestion, filtration, washing, drying, weighing, calculation The solubility product, the common ion effect Gravimetric calculations (key equations) . analysis Precipitation method Volatilization
established analytical methods we consider this term. Precipitation Gravimetry Gravimetric analysis is a standard classical method for determining the amount of a given component present in a host of solid and solution sample types. The method used here involves precipitating the component of interest from the unknown by means of an added reagent.
27. If a precipitate of known stoichiometry does not form, a gravimetric analysis is still feasible if we can establish experimentally the mole ratio between the analyte and the precipitate. Consider, for example, the precipitation gravimetric analysis of Pb as PbCrO 4. 14 (a) For each gram of Pb, how many grams of PbCrO 4 should form? Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . When the signal is the mass of a precipitate, we call the method precipitation gravimetry. . An accurate gravimetric analysis requires that the analytical signal—whether it is a mass or a change in mass—is proportional to .
Gravimetric Analysis 9/17/13 page 6 The gravimetric method is one of the most accurate methods of analysis. It is usually applied when the analyte concentration exceeds 1%. One distinct advantage is that no standard is required. However, precipitations may not be very specific (you may Gravimetric Analysis. Gravimetric analysis is a quantitative method in analytical chemistry wherein the concentration of a substance present in a sample is evaluated based on the measurement of its mass. This is done by precipitating the analyte (substance being analyzed) in a sample as a solid compound which is then separated, washed, dried and weighed.
- Optimize the precipitation conditions in order to obtain a desirable precipitate. - Learn how to do accurate measurents. . - Learn how to do calculations related to gravimetric analysis .What Is Gravimetric Analysis GRAVIMETRIC ANALYSIS In precipitation gravimetry, the analyte is converted to a sparingly soluble precipitate. This precipitate is then filtered, washed free of impurities, converted to a product of known composition by suitable heat treatment, and weighed. For example, a precipitation method for
Questions on Sulfate Analysis. Approximately how many mL of 5% BaCl 2 2H 2 O solution would be required to precipitate all the sulfate if we assume that your samples are pure sodium sulfate? Assume that the density of the barium chloride solution is 1.00 g/mL. If the samples were pure potassium sulfate would you require a smaller or larger volume of barium chloride solution .Gravimetric analysis is based on mass measurement. Q-11: What is the gravimetric analysis principle? Answer: Gravimetric analysis is based on the difference in masses of two analyte-containing compounds. The idea behind gravimetric analysis is that the mass of an ion in a pure compound can be calculated and then used to calculate the mass . Precipitation gravimetry continues to be listed as a standard method for the determination of \(\text{SO}_4^{2-}\) in water and wastewater analysis [Method 4500-SO42– C and Method 4500-SO42– D as published in Standard Methods for the Examination of Waters and Wastewaters, 20th Ed., American Public Health Association: Wash- ington, D. C., 1998].
Introduction Gravimetric analysis is a technique through which the amount of an analyte (the ion being analyzed) can be determined through the measurement of mass. Gravimetric analyses depend on comparing the masses of two compounds containing the analyte. The principle behind gravimetric analysis is that the mass of an ion in a pure compound can be determined and .
steps involved in gravimetric analysis
webReapers Slot. Play Reapers Demo Slot For Free ️ Cluster Pays ️ Print Studios ️ Maximum Win 20,000x ️ Best Online Casino Bonuses.
gravimetric analysis precipitation method|types of coprecipitation